
In cases where there MUST be positive or negative formal charges on various atoms, the most stable structures generally have negative formal charges on the more electronegative atoms and positive formal charges on the less electronegative atoms. formal charge valence shell electrons (free atom) lone pair electrons 1 2 bonding electrons (10.7.1) (10.7. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms.
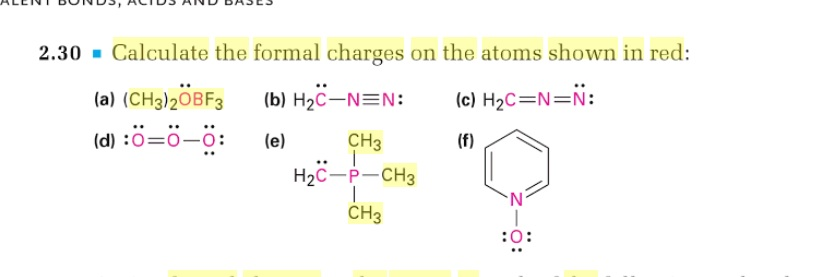
Typically, the structure with the most formal charges of zero on atoms is the more stable Lewis structure.


 0 kommentar(er)
0 kommentar(er)
